How kidney cancer can come back after surgery
Even after kidney cancer surgery, you may be at risk of your cancer coming back. That’s because it’s possible that some cancer cells were too small to be detected and could have spread to other parts of the body, such as the lungs, bones, liver, or brain. These cancer cells can then grow into tumors. When this happens, it’s called metastatic cancer.
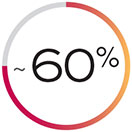
of patients at high risk of cancer coming back will have a recurrence within 5 years.
What determines your risk of kidney cancer returning?
There are many factors that determine your risk of cancer returning, including the results of your pathology report. So, it’s important to review these results with your doctor after surgery. He or she will use the pathology report to help determine if you are at high risk of recurrence, and if you are, discuss your options.
 LIVE SUPPORT: 1-877-744-5675
LIVE SUPPORT: 1-877-744-5675